Hydrogen Recovery Technology for Sodium Hypochlorite Generator
The brine electrolysis sodium hypochlorite generator is becoming more and more widely used in water treatment and disinfection due to its safety, easy availability of raw materials, and low operating cost. We know that the production of sodium hypochlorite(NaClO) solution by brine electrolysis also brings by-product hydrogen gas, which poses risks to environmental safety. Proper hydrogen treatment and discharge meet safety requirements, making it an ideal disinfection product; Produce 1kg of available chlorine, producing 0.35m ³. The larger the production of hydrogen gas in the equipment, the greater the hydrogen production, and a large amount of hydrogen gas is diluted and released into the atmosphere for waste. So the hydrogen recovery technology of electro chlorination generation emerged as the times require.
Process principle of chlorination system:
Sodium hypochlorite generator is a new type of equipment used for on-site production of sodium hypochlorite(NaClO) solution, suitable for various occasions that require chlorine disinfection.
The electrolysis process of the electrolytic salt water sodium hypochlorite generator is an electrochemical reaction process. The raw materials are salt+electricity+water, and the quality of the sodium hypochlorite(NaClO) solution is pure. Its working principle is that under a certain cell voltage, a sodium chloride solution undergoes a series of electrochemical reactions in the electrolytic cell, ultimately generating a sodium hypochlorite solution.
Reactions in the electrolytic cell:
(1) 2NaCl + 2H2O= 2NaOH +Cl2+H2
Anode reaction:2NaCl=> 2Na+ + Cl2+ 2e-
Cathodic Reaction:2H2O + 2e- =>H2+ 2OH-
The generated chlorine gas reacts with the generated sodium hydroxide solution to generate a sodium hypochlorite solution.
(2)Cl2 + 2NaOH = NaCl + NaClO + H2O
Interpolar reaction:2NaOH +Cl2 =>NaClO + NaCl + H2O
From the above two equations, it can be concluded that the main reaction of NaClO generation electrolysis is:
(3) NaCl+ H2O= NaClO+ H2↑
Total reaction: NaCl+H2O=>NaClO+H2
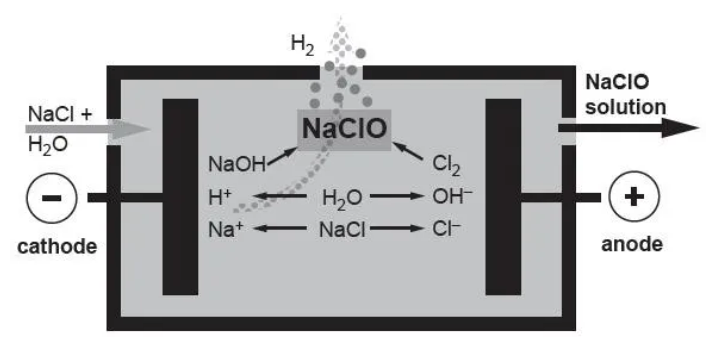
The process of generating sodium hypochlorite simultaneously produces a considerable amount of hydrogen gas. According to this calculation, approximately 0.35 liters of H2are generated for every 1 gram of NaClO produced.
The acquisition of hydrogen gas collection in the future will be a good thing for the country and the people; Not only can cheap disinfection products be obtained for water disinfection, but also selling hydrogen gas to generate profits will bring benefits to users who use electro chlorination generator.
https://bluewavv.com/e_products/Sodium_Hypochlorite_Generator-Salt-Water-6.html
Application
Contact Us

Name: Diana
E-mail: [email protected]
Skype: +86-15-22-27-71-011
WeChat: +8615222771011
Whatsapp: +8615222771011
Add: Office N.420D-C1 Tower Ajman,UAE









 Skype Chat
Skype Chat WhatsApp
WhatsApp  Mail inquiry
Mail inquiry
